States of Matter
Deepak Dhar
Department of Theoretical Physics,
Tata Institute of Fundamental Research,
Homi Bhabha Road,
Mumbai 400005, India
Abstract
This is a written version of a popular science talk for school children given on India's National Science Day 2009 at Mumbai. I discuss what distinguishes solids, liquids and gases from each other. I discuss briefly granular matter that in some ways behave like solids, and in other ways like liquids.
Introduction
"It is well-known that matter exists in three forms: solid, liquid and gas."
I picked this quotation from an NCERT textbook for grade XI, but I could have picked a different textbook, and found a similar statement there. It seems clear and uncontroversial enough, and has been repeated many times. What I would like to do in this lecture is to re-examine critically this commonly-accepted wisdom. In the process, we will learn something about the different states of matter.
However, telling you more about properties of solids, or liquids, or gases is not my main aim. What I really want to do is to impress upon you not to accept any such statement without thought. In fact, if you bother to think for 5 minutes, all of you can realize that it is not fully correct. The student should be able to think for himself/herself, and ask if the matter being taught is correct or incorrect. And if it is correct, to what extent? So that is my real aim. But we will do it by example, by discussing states of matter.
Let us start by asking why is it that there are three states of matter. It is a question like: Why do we live in three-dimensional space? Why there are three generations of quarks? and so on. And with a bit of thought, you will realize that the correct answer in this case is that states of matter is a classification scheme, like filing cabinets. There are a lot of materials, and we choose the grouping scheme that is most convenient. Other possible classification schemes could be alphabetical (e.g. in a dictionary), or based on some common properties (zoological classification of different animal species) or some mixture of these (e.g. books in a library). Materials can be classified based on color, or electrical properties, or whether they are organic or inorganic, or conductors or insulators, etc. All these classification schemes are useful, and are used when convenient.
Once we recognize the fact that different states of matter are like filing cabinets, the number of cabinets is purely a matter of convenience. One can always divide a class into smaller classes, or merge smaller classes into a bigger class. So, the number of different states of matter is not a deep question at all: it is whatever we want it to be.
Sometimes this sort of discussion ends up being a discussion about words, i.e. what is the dictionary definition of solid, liquid and gas? We are not discussing words. We are discussing the ideas behind the words. You may say, "Oh, this is a colloquial word. It doesn't have a very precise meaning". But lots of colloquial words have been adapted with precise meanings in science. Words like force, work, pressure, in ordinary language, can mean a lot of different things. For example, you can speak forcefully, or apply political pressure. However, in science, the meaning has been restricted to something quite specific, and you can quantify it as so many Newtons etc. So we would expect that even a common word like 'solid' can be given a specific meaning in science. Can we do that? And when we do that, what does it mean?
Let me clarify at the outset that 'states' of matter is not the same as 'phases' of matter. So you can have magnets, for example, and if you heat them, they become non-magnetic. There is a phase transition from magnetized phase to non-magnetized phase, but it remains a solid throughout. So you have a change of phase but not a change of state. So the 'state' of matter is a more general notion than 'phases' of matter and we are not going to discuss the latter. Also, we will restrict our discussion to simple materials. Things like salads are complicated and not the same everywhere: different parts are different. We are going to discuss only the simpler homogeneous matter.
The rest of the lecture is organized as follows. First we will discuss classification schemes. Then I will argue why liquid and gases should be treated as one state of matter. Then, we will look at differences between solids and fluids, and discuss different possible definitions of solids. I shall then discuss materials which are different from both solids and fluids, and are better treated as a separate state of matter. For lack of time, I will discuss only one of these, namely powders, and mention some of their unusual properties. And finally summarize our discussion.
Requirements of a good classification scheme
The first requirement of a good classification scheme is that the number of classes should be moderate: If you have 500 classes, that is not so useful. Each of these classes can be broken up into subclasses, if needed. That is what is done with animal classification, and with books in a library. But to begin with, you want a moderate number of classes.
Second, there should be a clear and unambiguous definition. If you give me an object, I should be able to tell which box it belongs in. There should be no confusion whether this is going to be called a solid, or a liquid or a gas. It should be a Yes/No answer. No in-betweens.
Thirdly, ease of classification. One should not have to spend a lot of effort in trying to decide to which class a given object belongs. You will have to do some test to decide. It is better if the tests do not involve expensive, not-easily-available apparatus.
The fourth requirement is naturalness and usefulness: I could have listed these separately, but I actually put them together because they are, in effect, the same thing. 'Natural' means it should not be arbitrary or artificial. For example, I can say that something is solid if its density if more than √2, in some units. This will be an unnatural and artificial definition (Why this value?). Artificial and unnatural definitions are not useful, as there is a good chance somebody else will choose a different number. Then it could be that in India, object A is a solid, but not in China. If you make an arbitrary definition, it is unlikely to be useful: the object has similar behavior whether it is above or below the legal threshold.
The characterization of solids, liquids and gases is known since ancient times. If you are going to the market to buy oil, it helps to know that you have to take a bottle along to bring back in. The essential difference between solids and liquids is qualitatively how you handle them. Can you just pick the matter with bare hands, or do you have to use a spoon? The classification in terms of solid, liquid and gas is in terms of the mechanical properties of matter.
Liquids and gases
Figure 1 gives what is called the phase diagram of a typical material, say water. Temperature is plotted along the X-axis and pressure on the Y-axis. For a particular temperature and pressure, the matter exists in a particular state, marked there tentatively as solid, liquid and gas. So, if you fix some pressure and increase the temperature slowly, initially you start with solid, which melts to a liquid, and then boils to form a gas. However, it is found experimentally that above a particular pressure if you heat the liquid, there is no sharp boiling point. The material just keeps on getting hotter, without any sudden change. So there is a critical point above which liquid and gas are indistinguishable. If I color the areas blue, green and red, I will have a hard time deciding where to put the boundary between the green and red regions. Any choice one makes would be arbitrary. In other words, liquids and gases are the same state. And together, they are called the fluid state. This is actually well-known, but the school textbooks continue to preach that there are three states of matter.
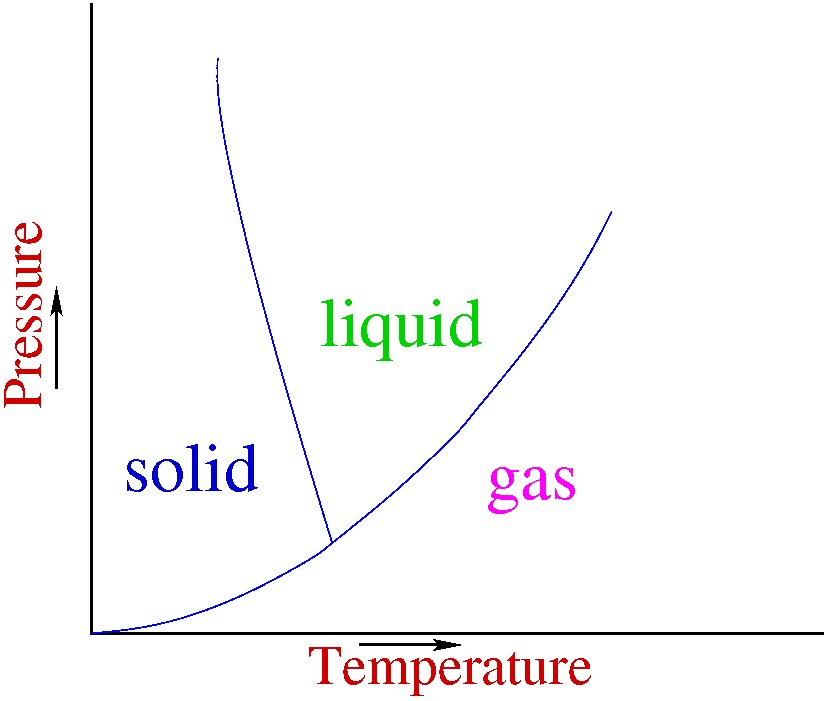
Figure 1: Phase diagram of a typical material showing solid, liquid and gaseous states
Thus, I have reduced my problem from three states of matter to two. Sometimes people say that plasma is the fourth state of matter. What is plasma? If you take a gas and heat it, more and more of the atoms become ionized as the temperature increases. When the material is very heavily ionized, it is called 'plasma'. Plasmas respond strongly to electric fields. However, again, between gas and plasma, there is no sharp point of transition. Ionization increases smoothly as you increase temperature. So any boundary between gas and plasma will be an arbitrary boundary, and not a natural distinction. And hence plasma and gaseous state are not distinct: they are the same state.
Solids and fluids
Let us now see how we can distinguish solids from fluids.
Def. 1:"Solids have a fixed shape and fixed volume but liquids have a fixed volume but no fixed shape, while gases have neither fixed shape nor fixed volume."
This is a definition that I remember from my schooldays. Perhaps it is in your textbook also. But let us look at it more closely. In Fig. 2, I have shown two pictures of a ball ( made of say rubber, half colored red, half green): Clearly, the ball gets a little squashed when you put it on the table and if you flip it, it is again squashed, but the shape is changed!. So, is it a solid or not a solid? In fact, all materials have finite compressibility, and will deform somewhat under force.
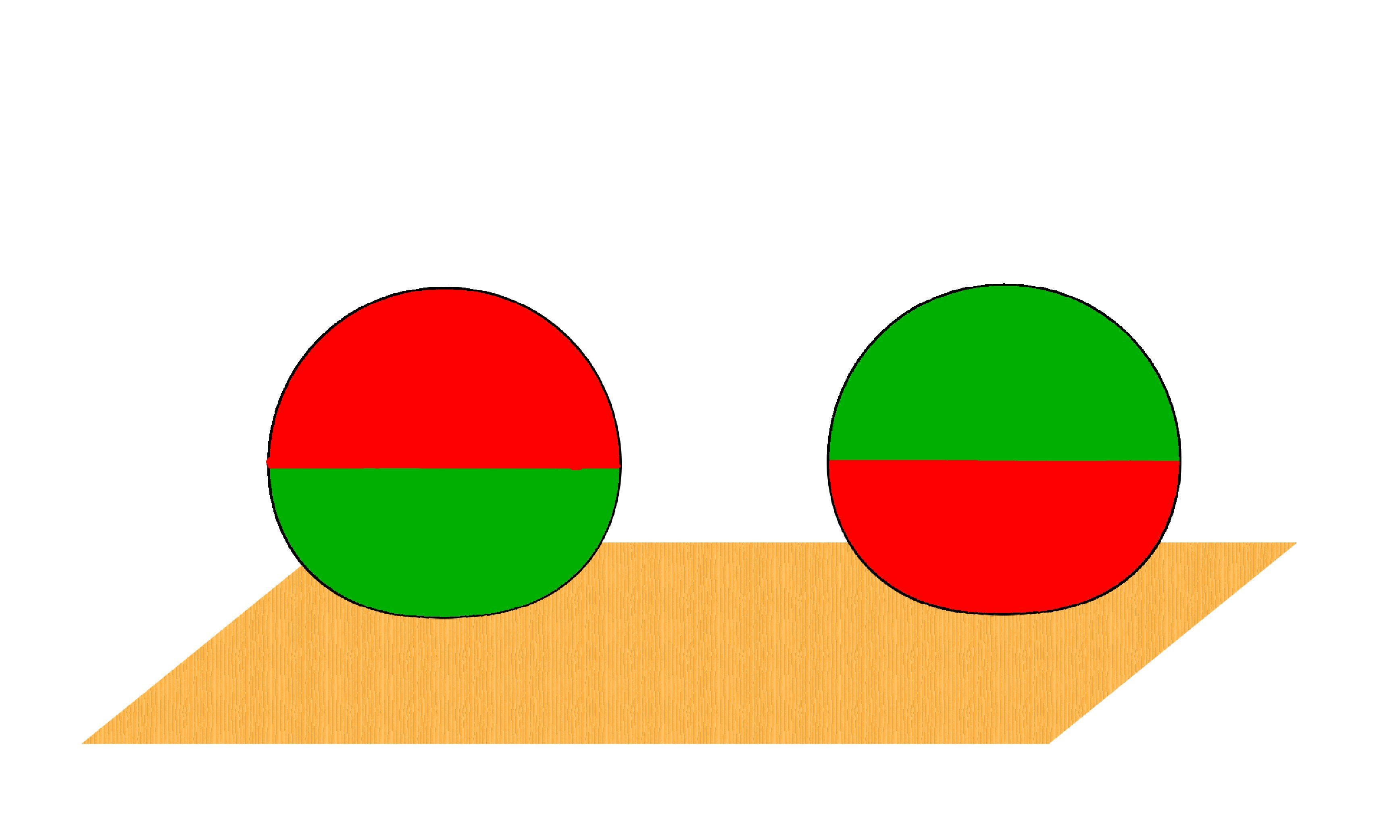
Figure 2: Solid balls will deform under gravity.
Let us try an alternate definition:
Def. 2: "In solids, the atoms vibrate about their mean positions, but in fluids, they move over all available space."
This poses a bit of a problem because it refers to molecules I cannot see with the naked eye. But if you take an atom in a solid, mark it in some way and observe its movement, then one would see, over time that it actually jiggles over larger and larger distances. So all particles diffuse in time and this diffusion constant is finite. The mean square displacement <R2> of a tagged particle is expected to linearly increase with time: <R2>~Dt.
The diffusion constant D is finite. In solids, an atom moves about 0.0001 mm in one minute, in liquids about 1 mm, and about 100 mm in gases. So it is not correct to say that, in solids, atoms do not move over all available space. They would, if you wait long enough.
Let us try another definition:
Def. 3:"Solids have a long range ordered periodic arrangement of atoms. Fluids have only a short range order."
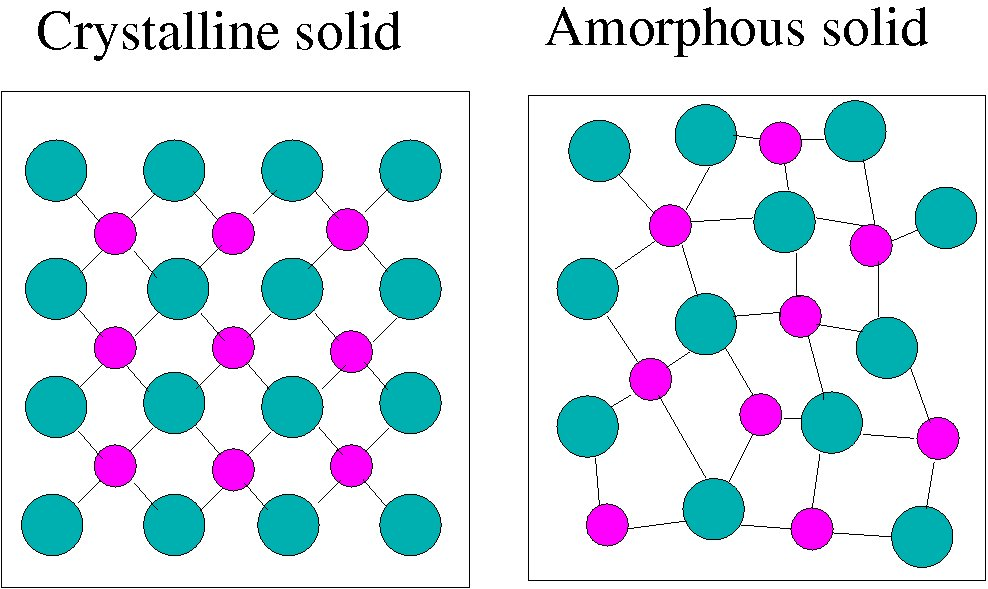
Figure 3: Schematic representation of the atomic arrangements in crystalline and amorphous materials.
In solids you expect a regular, periodic arrangement and in liquids, you have an irregular arrangement. So first, there is thermal motion in atoms; they are not in fixed position, they are jiggling around. Any time you take a snapshot, you will not find this nice periodic arrangement shown above. Each atom will be slightly displaced. So how does one distinguish this from the rest? There is a technical way of distinguishing it which says that take an x-ray diffraction picture of this. If you get sharp peaks, then there is an overall periodic structure. If there are no sharp peaks, then it is not a long-range periodic ordered structure.
However, if you say that long range periodic ordered structures are solids and other are not, plastic or some amorphous material like window glass, will not qualify as solids. So this definition is also not a good definition. There is another definition which says:
Def. 4: "Solids have a finite shear modulus, liquids do not."
Maybe you have not seen that definition above but that is the one most physicist like the most. What is the shear modulus? Suspend a weight from a wire from the ceiling. if you apply a twist to the weight, and let go, it starts to oscillate, and you get a torsion pendulum. If there was no restoring force which tries to undo the twist, it would not oscillate. Now suppose you have two cylindrical pipes one inside the other, you fill some liquid between these and then you apply a twist to the inner pipe. In this case, there is no restoring force. So there is no restoring force to shear in liquids, but it is in solids. This is the usually accepted distinction between solids and liquids.
However, it also has a problem because, what happens is that if you take a solid and you apply this twist, and you hold it for a long time, the force felt by the rod slowly decreases with time. This is called creep in solids. The material re-adjusts under this strain and the molecules move to relieve this strain. Thus how much shear modulus or how much restoring force there is, depends on how much time you wait before you measure it. And so if you take a really long time then may be it does not feel any force.
The definition of shear modulus involves very slowly changing forces, and it would appear that if you really wait very long, the shear modulus is always zero.
I was looking up other possible definitions for distinctions between solids and liquids and there is one which is not so often used in school textbooks but it was used as a distinguishing characteristic for materials in our ancient books. ( ).
Def. 5:"Solids can be cut with a knife, fluids not."
This also looks like a reasonable characterization. A tougher solid is harder to cut, but eventually you can cut it. For a liquid like water, there is no use trying to cut it with a knife. Even this definition turns out to be not very useful because there are things like cold welding of solids. You can take two solids, you can cut them, put them on top of each other, vibrate them a little bit and they become the same again. This is called cold welding. In the liquid, you cut it with a knife and the two separated parts behind the knife's moving edge re-join again. This self-healing after the cut can occur in solids as well as liquids.
This is becoming a bit confusing. To recall, we started by saying that one of the requirements for a good classification scheme was a clear, unambiguous definition. It seems reasonable to expect, but now, we find this difficult to satisfy.
In fact, 'solid-like' and 'fluid-like' behavior is a matter of length and time scales. A small drop of water or mercury resists change of shape, and is quite "rigid". A "ductile" metal flows at long-enough time scales. Falling from a plane on a lake, you are likely to break your bones, as badly as falling on hard ground. A single brick is clearly a solid; a truck-load of bricks can be poured out, and acquires fluid-like properties.
Powders: a different state of matter
Powders are granular materials like sand, wheat, flour. I would now like to argue that they are a state of matter different from both solids and fluids. Firstly, one can broadly distinguish between two types: wet and dry powders. In the following, I restrict myself to the former.
Firstly, powders can be poured from one vessel to another, and take the shape of the vessel. In this sense, they are like fluids. However, if pour a powder on a flat table, from a point above, they form a conical pile, in which the slanting surface makes a finite angle with the horizontal. This angle is characteristic of the material, and is called the angle of repose. If powders were fluid, this angle would have been zero.

Figure 4: A small sandpile on a flat table.
In general, the flow of powders is very different from fluids. While the latter is quite well understood, and is described by laws of hydrodynamics, even the equivalent of hydrostatics for powders is only partly understood. For example, consider a long cylindrical vessel, which is filled with sand or water up to a height $h$. In the case of fluids, the pressure at the base of the vessel increases linearly with the height of the column. In case of sand, it initially increases, but tends to a finite saturation value even as the height is increased [Fig. 5].
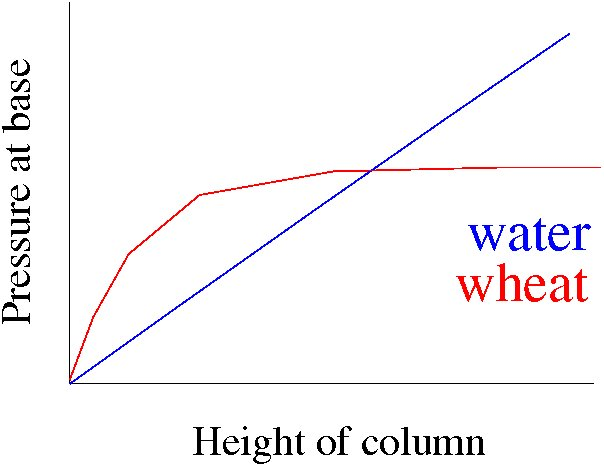
Figure 5: Pressure at base of a cylinder filled with water and sand, as a function of height of the column.
Another interesting behavior of powders is called the "brazil-nut effect". This refers to the fact that larger heavy particles rise to top in shaken granular media. The name refers to the well-known phenomena of larger nuts being found near the top in a shaken cereal box. I understand that the same phenomena can be seen with large cannonballs in a shaken box of sand. In liquids, the heavier cannonballs would be expected to sink, not rise. Clearly, the well-known law of Archimedes is not valid for powders.
This coming together of larger particles in shaken mixtures has important consequences. In many applications, it is important for powders to be well-mixed, e.g. in making medicine tablets. But, if size separation occurs, keeping a well-mixed granular mixture in a rotating drum would make it unmixed.
Other states of matter
There are many other types of matter whose behavior differs substantially from solids and fluids, so that it seems reasonable to classify them as separate states of matter. A detailed discussion of each of these would require too much time. I only mention them briefly here. You can learn more about them from books and from the internet.
For example, glasses, like window glass, look like solids, but their atomic structure is like that of liquids, and they seem to flow, though very slowly. They can be thought of as very viscous liquids, but perhaps better thought of as a distinct state of matter. Liquid crystals, the stuff used in your mobile phone displays, flow like fluids, but show partly crystalline atomic order, and can be thought of as a state of matter between solids and fluids. These are 'solid -like' at atomic level, but 'liquid-like' in bulk behavior, while in glasses, the converse happens. Helium at low temperatures when it becomes a superfluid, or Bose -Einstein condensates are rather exotic forms of matter, not encountered in everyday life, but they have very unusual flow properties and certainly qualify as distinct states of matter. Recently, there has been some indication of experimental evidence of a state called 'supersolids', that have periodic atomic arrangement in space, but flow like superfluids. Then there are colloids, gels, emulsions, foams... These could also be considered as different states of matter, but one can argue that they are not really homogeneous. Away from earth, in neutron stars, one has matter in the form of neutrons, and that is certainly a new state of matter. Astrophysicists these days even speak of 'dark matter', which, if it is found to exist, is going to be very different from other known forms of matter.
Summary
To summarize, the main thing I have tried to emphasize in this talk is the importance of thinking for yourself in whatever you study. Another is that we should correct our textbooks. Sometimes, the changes required are not so large, and some textbooks do say things correctly. For example, the textbook "Advanced Chemistry" by P Mathew, (Cambridge Univ. Press) says: "Almost all substances fall neatly into one of the three categories: solid, liquid and gas…."
I argued that it is difficult to give a clear precise definition of different states of matter: It is a question of length and time scales. The surface of water is hard, solid-like for large velocity impact, and ductile metals flow (can be pulled into wires). Powders are examples of states of matter different from both solids and fluids.
And, finally, the students should realize that there is much we do not understand, even about everyday life objects. Understanding things better can be very exciting. It is good to remember that today, on our National Science Day.